CDC advisers to vote on dropping universal newborn hepatitis B vaccine
Written by ABC Audio ALL RIGHTS RESERVED on September 18, 2025
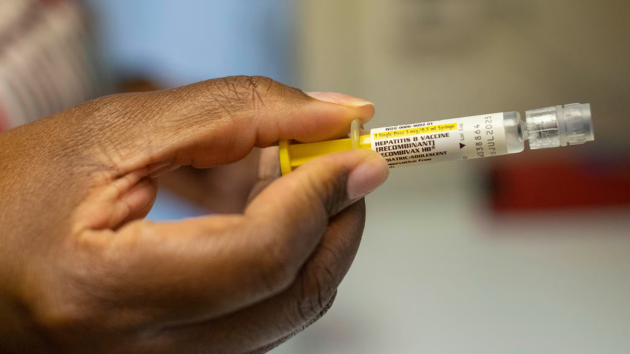
(WASHINGTON) — An influential group of advisers for the Centers for Disease Control and Prevention during a meeting on Thursday is considering dropping a longstanding recommendation to give all newborns a hepatitis B vaccination in the hospital.
The CDC vaccine advisory committee, called ACIP, is also weighing new restrictions on existing recommendations for the combined MMRV shot to protect against measles, mumps, rubella and chickenpox.
An official vote is expected on Thursday afternoon. From there, the vote will need signoff from the acting CDC director or the Health and Human Services secretary.
The committee is not considering eliminating or recommending against these vaccines completely. But the changes that have been proposed could result in major disruptions and more illness, experts warn.
Experts say these changes could cause confusion, more doctors’ appointments and more individual shots for children, which could potentially lead to missed cases or more infections. It could also complicate vaccine supply and manufacturing logistics.
On Wednesday, Republican Sen. Bill Cassidy, a liver doctor, told reporters that Americans should not have confidence in this committee’s decisions if they change the current vaccination schedule.
“I can promise you there will be some hepatitis B transmission,” Cassidy told reporters when asked what would happen if the committee makes changes to already-existing recommendations.
This week’s meeting is the committee’s second since Health and Human Services Secretary Robert F. Kennedy Jr. dismissed all 17 of its members in June. Of the 12 advisers who have since been appointed, many have previously expressed vaccine-skeptical views.
Most major insurance providers have said they will continue covering existing vaccines at least through 2026. But any changes made today will likely impact the more than half of American children who are funded through a federal program, which is tied to the CDC committee recommendations.
The committee members so far are not unanimous during discussion, with some saying the proposed changes could take away parents’ choice — especially regarding the combined MMRV vaccine.
Currently, parents have a choice of giving their children measles, mumps, rubella and chickenpox all as one shot, or they can opt to give measles, mumps and rubella as one shot, and give chickenpox as a separate dose. Some studies have suggested a slightly elevated — but overall very rare — risk of seizures when all four are given as a combo shot to kids 12 to 15 months old.
But many parents and clinics may still prefer a single shot. Children also get a second dose of MMRV after the age of 4. The upcoming vote Thursday only discusses the first dose, and parents would still have a choice about their preference for the second shot in older children.
“The disadvantage of giving two doses, or as was suggested, separating the two doses, is that we know compliance falls, and the advantage of combination vaccines is that children and adults are more likely to complete the vaccine requirements if it’s given as a single dose,” said ACIP member Dr. Cody Meissner.
“If parents would choose to have one one jab and one vaccination, it would not be covered by the [federal vaccines for children program] over time accessing clinical care, if they understand the risks and benefits, that option is basically taken away from them,” said Dr. Joseph R. Hibbeln, another committee member.
Copyright © 2025, ABC Audio. All rights reserved.





